Background
Nucleic acid therapeutics, now and future
In addition to the low molecular weight and antibody drugs, the nucleic acid therapeutics have become a new drug modality and raise hopes for patients and families with diseases that have not been treated before. The target organs and tissues are now limited to the liver, muscle or spinal cord and expected to expand to wider range of disease.
Importance of technology to study in vivo pharmacokinetics and biodistribution
For nucleic acid therapeutics to expand their application to diseases, it is desirable to distribute to the target organ at an appropriate dose to increase the therapeutic effect and minimize side effects. Micro-dosing technology may provide necessary information about the biodistribution and pharmacodynamics of the nucleic acid therapeutics with only a limited risk to patients.
Imaging technology is needed for the development of new nucleic acid therapeutics
Micro-dosing technology such as by PET imaging using 18F-labeled drugs is now commonly applied to study biodistribution and pharmacodynamics of small molecular drug candidates and contributes to speed up the development of drugs. However, nucleic acid therapeutics lacks suitable micro-dosing technology. Prof. Sasaki' group of Kyushu University has developed an innovative technology that can chemically modify the desired position of nucleic acid with various functional groups including radioisotopes. Micro-dosing test may be possible using radiolabeled nucleic acid therapeutic candidates, which should greatly accelerate the clinical trial processes.
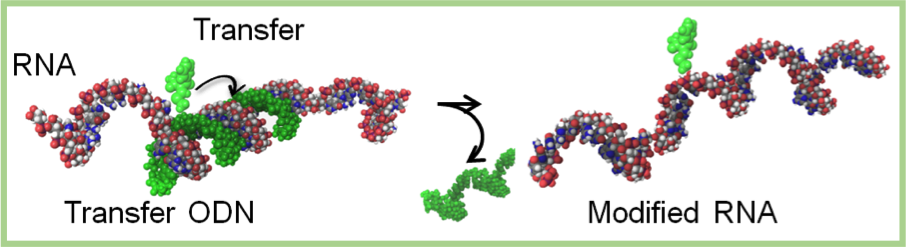
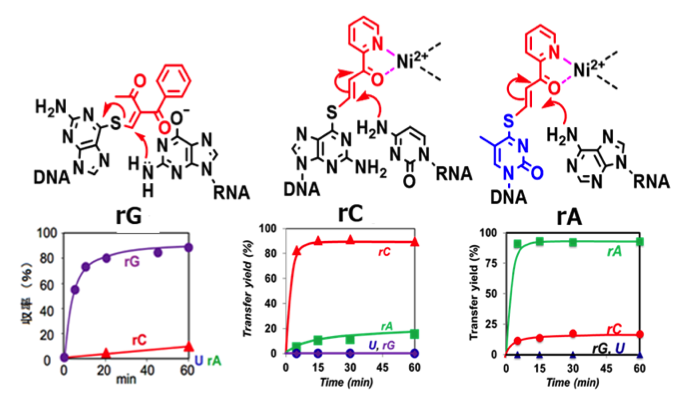
Key technology: sequence- and base selective radio-labeling
Reaction scheme and its selectivity
- 1.The transfer ODN is designed based on the target RNA sequence.
- 2.The transfer ODN is hybridized with RNA and the transfer group migrates to the target base of RNA.
- 3.The transfer group is reduced with NaBT4 to produce site-selectively radio-labeled RNA
- 4.This key technology is applied to 3H-labeling, and radiolabeling with short half-life such as 11C, 18F, 123I, and 125I will soon be established.
Characteristics and benefits
- 1)Post-synthetic labeling technology.
- 2)Guanosine, cytosine and adenosine at the predetermined position are selectively labeled.
- 3)Neighboring bases are separately labeled.
- 4)A target base of long RNA such as mRNA can be also selectively labeled.
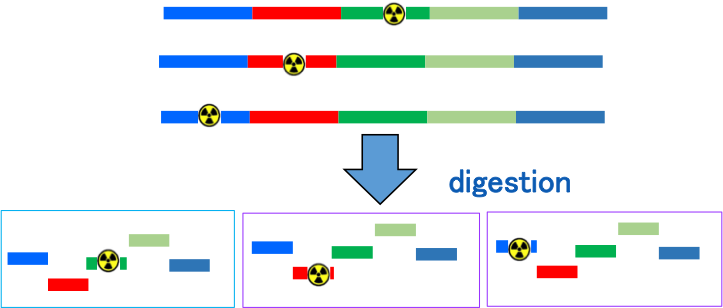
Illustration of internally labeled nucleic acid for pharmacodynamics study
Analysis of each fragments
Nucleic acid therapeutics suffer from metabolism to form many fragments. Tracing differently labeled nucleic acids provides the fate of each fragments.
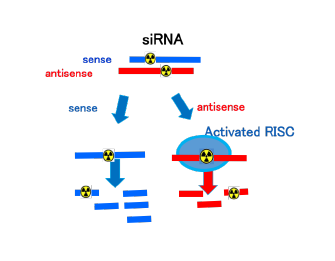
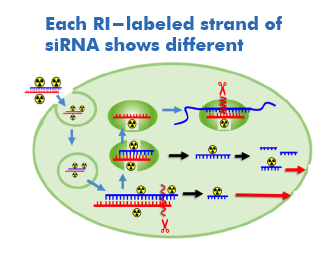
Different fates of RI-labeled strands of siRNA
Each fate of the sense and antisense strands of siRNA is investigated using internally labeled strands.
References
- Development of Novel Functional Molecules Targeting DNA and RNA. Shigeki Sasaki, Chem. Pharm. Bull. 67, 508-518 (2019). doi.org/10.1248/cpb.c19-00169.
- Site-specific modification of the 6-amino group of adenosine in RNA by an inter-strand functionality-transfer reaction using an S-functionalized-4-thiothymidine, Oshiro I. Jitsuzaki D. Onizuka K. Nishimoto A. Taniguchi Y. and Sasaki S., ChemBioChem, 16, 1199-1204 (2015). doi.org/10.1002/cbic.201500084.
- Remarkable acceleration of a DNA/RNA inter-strand functionality transfer reaction to modify a cytosine residue: the proximity effect via complexation with a metal cation, Jitsuzaki, D. Onizuka, K. Nishimoto, A. Oshiro, I. Taniguchi, Y. Sasaki, S., Nucleic Acids Research, 42, 8808–8815 (2014). doi.org/10.1093/nar/gku538.
- The oligodeoxynucleotide probes for the site-specific modification of RNA, Sasaki S., Onizuka K. and Taniguchi Y, Chem. Soc. Rev., 40, 5698-5706 (2011). doi.org/10.1039/c1cs15066a.
- Pin-Point Chemical Modification of RNA with Diverse Molecules through the Functionality Transfer Reaction and Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction, Onizuka K, Shibata A, Taniguchi Y, Sasaki S., ,Chem. Comm.,47, 5004-5006 (2011). doi.org/10.10389/C1CC10582E.
- Site-specific covalent modification of nucleic acids guided by functionality-transfer oligodeoxynucleotides, Onizuka K, Taniguchi Y., Sasaki S., Bioconjugate Chem. 20, 799-803 (2009). doi.org/10.1021/bc900009p.